Nanotechnology & materials
Vivian Ferry
She uses nanocrystals to trap light and increase the efficiency of solar cells.
Photo by Hunt and Capture
Global
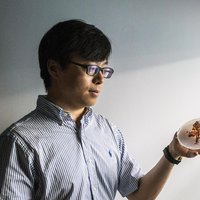
Yihui Zhang
Pop-up nanostructures make it far easier to fabricate very tiny shapes.

Asia Pacific
Madhu Bhaskaran
Transparent Wearable Devices

Asia Pacific
Javier Gomez Fernandez
Shrilk and Bioinspired Materials: The Future of Sustainable Manufacturing

Europe
Timothée Boitouzet
Translucent wood: a resistant and sustainable material for constructing the buildings of the future
