Nanotechnology & materials
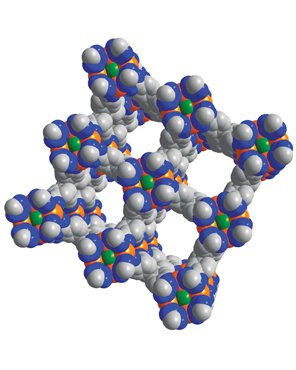
Mircea Dinca
Using sponges to improve and store alternative fuels
Courtesy of Mircea Dinca

Europe
Ana Díez
Nanotechnology for building better materials in aerospace

Latin America
Nadim Morhell
A new diagnostic tool to measure neonatal blood viscosity

Global
Baile Zhang
A new type of invisibility cloak made from a common material can work with larger objects <br>

Europe
Rafael Luque
Nanoparticles from residues used to turn biomass into bioplastics and biofuels